Alcohol tunes the switching behavior of responsive polymers
A new approach involves neutron scattering experiments and theoretical physics
2016-03-02 – News from the Physics Department
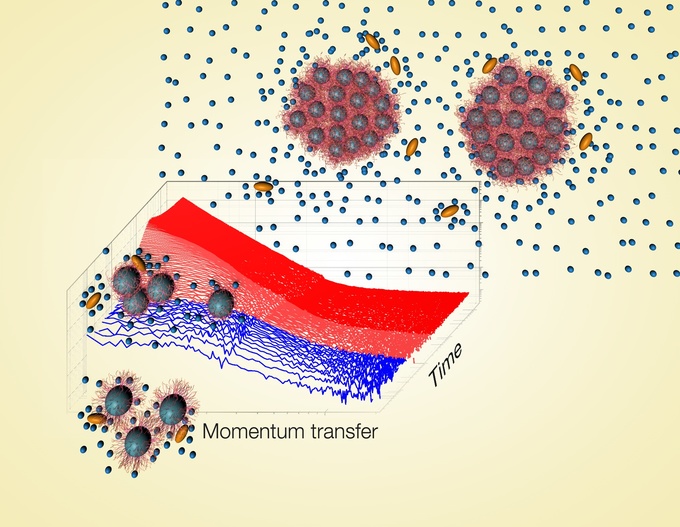
The back cover of the current issue of “Macromolecular Rapid Communications” features experiments on polymer colloids obtained by a collaboration of Prof. Christine M. Papadakis from the Physics Department of Technical University of Munich with colleagues from the universities of Potsdam and Cambridge, UK, and from the Institut Laue-Langevin in Grenoble, France.
Colloidal dispersions of soft matter particles in aqueous media are ubiquitous. Cow milk, for instance, constitutes an important colloidal emulsion of fat in water. Technical applications of colloids include water-based paints and medical drug delivery systems. Colloids may also be formed by the association of macromolecules like polymers dissolved in liquids. In order to control the formation of these colloids, it is important to understand the physical mechanisms behind their formation. However, not only the complexity of the colloid-forming molecules must be considered, but also the complex interactions of the solvent, which is especially important in water. Only this allows a quantitative assessment and understanding of the underlying interactions.
Fascinating properties of dispersed polymers upon a temperature jump
In an international collaboration, physicists of TUM have studied the aggregation of polymers using poly(N-isopropylacrylamide) (PNIPAM). PNIPAM is water-soluble, i.e. hydrated, below its cloud point of ~32 °C. At higher temperatures, the polymers change conformation from a hydrated state to a dehydrated, collapsed state and form large aggregates. Due to this drastic change upon a small temperature change, the polymer is called “thermoresponsive”. These properties are of great interest for a potential use in biomedicine and in microfluidics; therefore PNIPAM is one of the most intensely studied thermoresponsive polymers today. At the core of the research efforts is the characterization and quantification of the aggregation process, which is intimately linked to the interaction energy between the macromolecules.
The present study investigates self-assembled micelles from diblock copolymers featuring PNIPAM and a short hydrophobic block from another polymer, polystyrene (PS). Since PNIPAM is readily dissolved in water at room temperature, whereas PS is not, globular micelles are formed, where the PNIPAM shell shields the PS core from the water solvent. The micellar dispersions were prepared in water or in mixtures of water and ethanol or methanol – two alcohols of different molar volume. A dedicated sample environment allowed fast temperature jumps across the cloud point of these dispersions. The subsequent aggregation process was observed by time-resolved small-angle neutron scattering experiments, carried out at instrument D22 at the Institut Laue-Langevin in Grenoble, France.
“Only by means of the excellent sample environment provided by ILL, we could realize these fast changes of temperature and follow the aggregation process with an unprecedented time resolution”, Christine Papadakis points out. It turned out that the growth rate increased with the size of the alcohol molecules added to the dispersion.
Tailor-made solvation behavior of colloids
Prof. Alessio Zaccone – who recently moved from TUM to the University of Cambridge – collaborated on the data analysis. He has recently developed a model that relates the growth rate to the interaction energy between the aggregates. It turns out that the presence of alcohol molecules significantly alters the interaction energy. Thus, the addition of alcohol can be exploited to fine tune the self-assembly process of thermoresponsive systems for certain applications.
“The alcohol molecules disrupt the highly structured hydration layers on the residual hydrophilic segments of the aggregate surface, thus reducing the repulsion due to the hydration layers and accelerating the aggregated growth,” Alessio Zaccone explains.
The combined effort of advanced experimental and theoretical methods leads to a better understanding of molecular interactions in responsive, aqueous systems and thus to fine-tune their switching behavior. Apart from the case at hand, the approach may also be applicable to the understanding of self-assembly of biomolecules.
- Editing
- Dr. Johannes Wiedersich
Publication
Links
Contact
- Prof. Christine Papadakis,
- Physik-Department, Technische Universität München,James-Franck-Str. 1, 85747 Garching, GermanyTel.: (+49) 89 289 12 447, Fax: (+49) 89 289 12 473E-Mail: papadakis@tum.de